Hyphema
All content on Eyewiki is protected by copyright law and the Terms of Service. This content may not be reproduced, copied, or put into any artificial intelligence program, including large language and generative AI models, without permission from the Academy.
Disease Entity
Hyphema of iris and ciliary body:
- ICD-9-CM diagnosis code: 364.41
- ICD-10-CM diagnosis code: H21.0
Disease
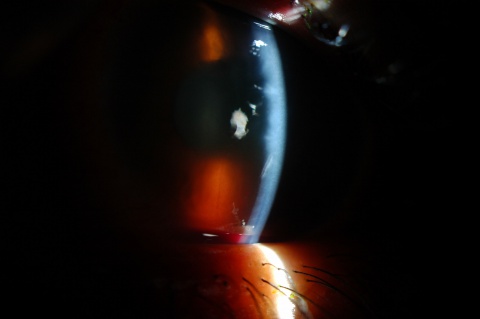
A hyphema is the accumulation of red blood cells within the anterior chamber. A small amount of blood that is only evident under close microscopic examination is referred to as a microhyphema. A majority of patients present with a history that correlates to the etiology. The most common risk factors are a history of trauma or recent ocular surgery. However, a seemingly spontaneous hyphema can result at times from other causes. Despite the degree or etiology, the management of a hyphema and its associated complications can be challenging for any ophthalmologist.
Etiology
A hyphema can occur after blunt or lacerating trauma, after intraocular surgery, or spontaneously in conditions such as rubeosis iridis, juvenile xanthogranuloma, iris melanoma, myotonic dystrophy, keratouveitis (eg, herpes zoster), leukemia, hemophilia, and von Willebrand disease. It can also occur in association with the use of substances that alter platelet or thrombin function (eg, ethanol, aspirin, warfarin).[1]
Blunt trauma is the most common cause of a hyphema. Compressive force to the globe can result in injury to the iris, ciliary body, and trabecular meshwork and their associated vasculature. The shearing forces from the injury can tear these vessels and result in the accumulation of red blood cells within the anterior chamber.
Hyphemas can also be iatrogenic in nature. Intraoperative or postoperative hyphema is a well-known complication of any ocular surgery. Rarely, the placement of an intraocular lens (IOL) within the anterior chamber can result in chronic inflammation, secondary iris neovascularization, and recurrent hyphemas, known as uveitis-glaucoma-hyphema (UGH) syndrome.[2] This is a direct result of a malpositioned or rotating anterior chamber IOL. This condition has also been reported with posterior chamber, sulcus, and suture-fixated IOLs.[2][3] Additionally, a hyphema resulting from an ocular laser procedure is a possible adverse event. Postlaser hyphema is not uncommon after the use of the Nd:YAG laser for a peripheral iridotomy. Typically, the resulting hyphema is minimal and self-limited.
A spontaneous hyphema, commonly confused with a traumatic hyphema, is likely secondary to neovascularization (eg, diabetes mellitus, ischemia, cicatrix formation), ocular neoplasms (eg, retinoblastoma), uveitis, or vascular anomalies (eg, juvenile xanthogranuloma).[4]
Diagnosis
Diagnosis is made with slit-lamp examination of the anterior chamber. A large hyphema can be noted with pen-light examination alone.
History
A majority of patients will have a history consistent with recent ocular trauma or surgery. However, in the setting of a spontaneous hyphema, further investigation may be required. It is important to ask patients if they have a past history of bleeding diathesis or anticoagulation therapy, which can be a rare risk factor in the development of a hyphema. It is also important to discuss factors that may predispose the patient to ocular complications of a hyphema, such as clotting disorders or sickle cell disease.
Sickle cell anemia is an especially important factor to consider. Red blood cells in this disease process can sickle in the anterior chamber, causing them to become rigid and unable to easily escape through the trabecular meshwork. This leads to a much greater likelihood of elevated intraocular pressure (IOP).[5] In addition, intravascular sickled red blood cells can cause catastrophic vaso-occlusive events such as central retinal artery occlusion and ischemic optic neuropathy, even at mildly elevated IOP that would not otherwise be a threat to most eyes. Sickle cell anemia is much more common in those of African descent, perhaps as high as 10%.[6] Even those with sickle cell trait are at risk, not just those with sickle cell disease.
Physical Examination
The examination for a hyphema should consist of a routine ophthalmic workup (ie, visual acuity, pupillary examination, IOP, slit-lamp examination) as well as gonioscopy to evaluate the condition of the angle and trabecular meshwork. This is important especially in the setting of ocular trauma to gain an understanding of the extent of the trauma. This can be delayed until after the critical 5-day, high-risk, rebleed period, particularly when doing dynamic gonioscopy. Angle abnormalities such as peripheral anterior synechiae (PAS) and angle recession may commonly be found.[4] It is also critical to measure the height of the hyphema from the inferior limbus.
Signs
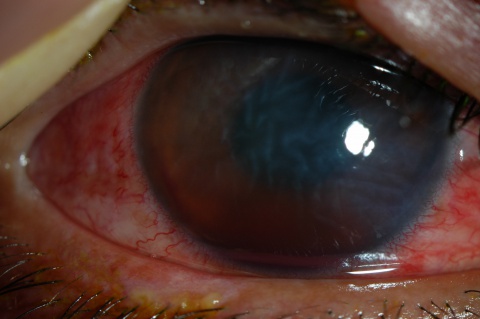
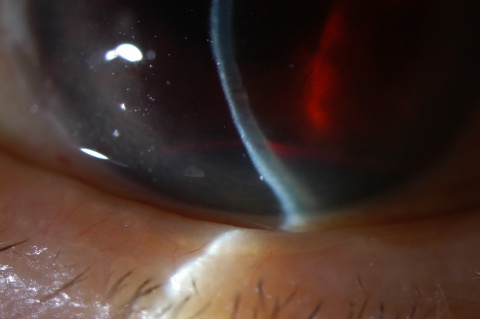
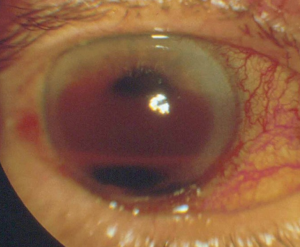
A large hyphema can be noted with pen-light examination alone. The height and color of the hyphema should be documented. Height can be measured in millimeters from the inferior corneal limbus. Color can vary from red to black depending on the time frame of the hyphema. Blood that has clotted will appear darker in appearance (black). It is important to evaluate intraocular pressure.
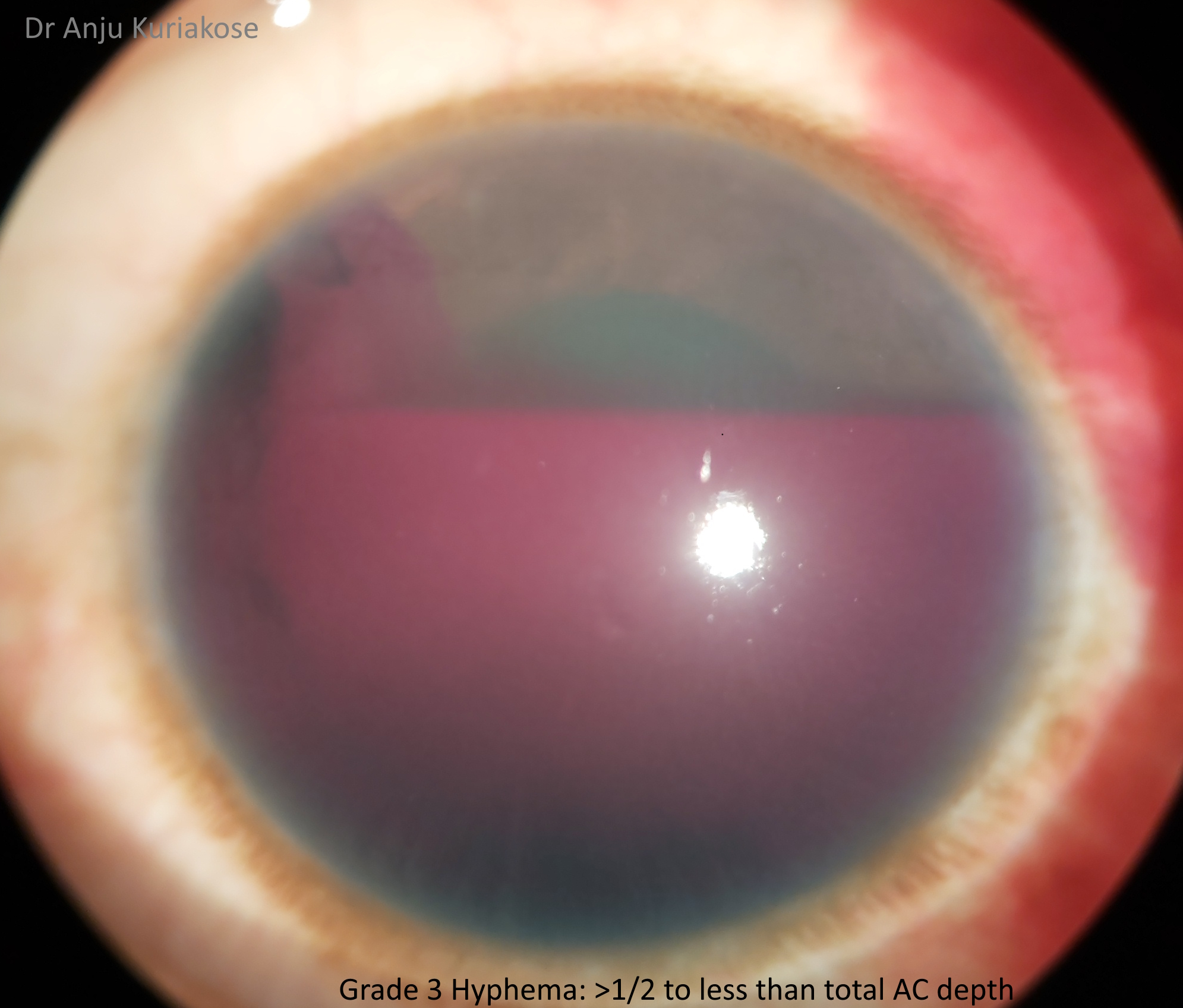
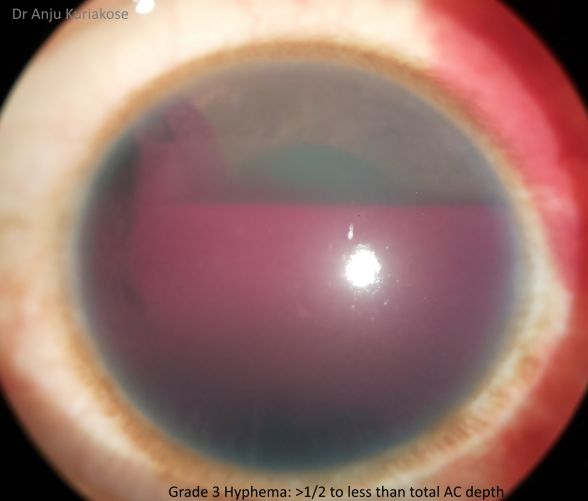
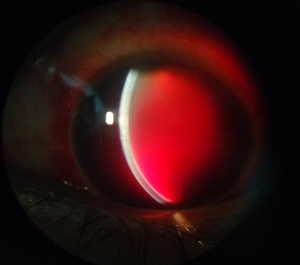
Hyphemas can be graded in the following manner:[4]
- Grade 0: There is no visible layering, but there are red blood cells within the anterior chamber (microhyphema).
- Grade I: Layered blood occupies less than one-third of the anterior chamber.
- Grade II: Blood fills one-third to one-half of the anterior chamber.
- Grade III: Layered blood fills one-half to less than total of the anterior chamber.
- Grade IV: There is total filling of the anterior chamber with blood. If the anterior chamber is completely filled with bright red blood, it is called a total hyphema. If the anterior chamber is filled with dark red-black blood, it is called a blackball or 8-ball hyphema. The black color is suggestive of impaired aqueous circulation and decreased oxygen concentration. This distinction is important because an 8-ball hyphema is more likely to cause pupillary block and secondary angle closure.[6]
Grading hyphemas has value because the risk for IOP elevation increases with larger hyphemas, with the exception being in sickle cell cases where even a small amount of red blood cells can elevate IOP. Coles, in his series of 235 cases, noted that the risk of elevated IOP went up dramatically for those whose hyphemas filled more than 50% of the anterior chamber, or a grade III hyphema. Only 13.5% of those with grade I to II hyphemas had an IOP increase, compared with 27% of those with grade III hyphemas and 52% with grade IV. Also, cases with rebleeding had a more than 50% chance of having elevated IOP.[7]
Symptoms
Symptoms associated with a hyphema can vary depending on the etiology. Typically, patients will complain of associated blurry vision and ocular distortion. In the setting of trauma or secondary IOP elevation, patients may complain of pain, headache, and photophobia.
Laboratory Test
All African American patients with a hyphema should be screened for sickle cell trait or disease with a sickle cell prep. A greater risk of trabecular meshwork obstruction with red blood cells exists while cells are in the sickled state. Resultant elevated IOP levels put the patient at greater risk of permanent vision loss from optic nerve damage. Hemoglobin electrophoresis can be used as a confirmatory test for a positive sickle cell prep.
Differential Diagnosis
Traumatic hyphema: Blunt trauma to the eye may result in injury to the iris, pupillary sphincter, angle structures, lens, zonules, retina, vitreous, optic nerve, and other intraocular structures. Blunt trauma to the eye is associated with a rapid, marked elevation in IOP with sudden distortion of intraocular structures.[8] As a result, normal vasculature within the anterior chamber is exposed to shearing forces that are responsible for hyphema formation.
Months to years following uncomplicated cataract surgery, abnormal neovascularization can also occur via an ingrowth of episcleral vessels into the incision site, which are fragile and vulnerable to tear.[9][10][11] Bleeding of these vessels in the anterior chamber is often precipitated by microtrauma to the limbal wound and presents as sudden blurring of vision and transient hyphema, a condition named Swan syndrome after Dr. Kenneth Swan. Such hyphema may resolve spontaneously; however, accumulation of blood in the lower angle can lead to angle-closure glaucoma.[1]
These recurring bouts of atypical anterior chamber bleeding and blurred vision are most commonly associated with a history significant for cataract extraction; however, Swan syndrome has also been noted in a case post-trabeculectomy.[9][12]
Gonioscopic examination is a useful tool for visualization of abnormal wound vessels, bleeding, and eventual diagnosis.
Hyphema secondary to ocular surgery or laser: Hyphema is a risk factor for any intraocular surgery performed; however, it is rare in routine elective cases. The incidence is higher in patients with a past medical or ocular history that predisposes them to irregular vasculature within the anterior chamber (eg, ocular ischemia and neovascularization).
Neovascularization: Abnormal blood vessel growth on the iris or ciliary body or within the angle may lead to the development of a hyphema. Typically, this neovascularization is a direct result of posterior segment ischemia. Retinal microvascular disease in diabetic patients is by far the most common etiology. Vascular endothelial growth factor (VEGF) upregulation from ischemic tissue disperses within the eye and allows for anterior segment neovascular growth. Retinal ischemia also can occur subsequently to retinal arterial or venous occlusion. Another cause of the neovascularization is carotid stenosis, which can lead to ocular ischemia (ocular ischemic syndrome).[4]
Neoplasm: Tumor growth is dependent on an extensive vascular supply. Ocular neoplastic disease such as melanoma (iris, ciliary body, and/or choroidal) or pediatric retinoblastoma can predispose patients to the development of a hyphema.
Inflammation/infection: Common intraocular inflammatory conditions that can result in neovascularization and resultant hyphema are herpetic (herpes simplex virus [HSV] and varicella-zoster virus [VZV]) uveitis and Fuchs heterochromic iridocyclitis.
Vascular anomaly: Juvenile xanthogranuloma (JXG) is a rather rare disease that is commonly thought of when evaluating pediatric patients with a hyphema. It is mainly a skin disorder characterized by a typically raised, orange lesion, occurring either singly or in crops, and regresses spontaneously with occasional involvement of the eyes. The most common ocular finding is diffuse or discrete iris nodules, which could be quite vascular and may bleed spontaneously, resulting in a hyphema.[13]
Management
Uncomplicated hyphemas should be managed conservatively, with an eye shield, limited activity, and head elevation.[2] Patients should be monitored closely during the first few days after injury as this is the highest-risk time frame for rebleeding (see below in the "Complications" section for more details on rebleeding). Maintaining head elevation of at least 45 degrees will allow the hyphema to settle inferiorly within the anterior chamber. This avoids central visual obstruction, as well as limits both corneal endothelial and trabecular meshwork exposure to red blood cells. Most patients can be managed in an outpatient setting with close follow-up to evaluate vision, hyphema regression, and intraocular pressure. However, hospitalization should be considered for noncompliant patients, patients with bleeding diathesis or blood dyscrasia, patients with severe ocular or orbital injuries, and patients with concomitant IOP elevations with known sickle cell disease.[14] Pain control for all patients with a hyphema should not include aspirin-containing products or nonsteroidal anti-inflammatory drugs (NSAIDs), as these can increase the risk for further bleeding.
Medical Management
Medical treatment for an isolated hyphema typically is topical. Topical corticosteroids (systemic for severe cases) may reduce associated inflammation, although the effect on the risk for rebleeding is debatable. Topical cycloplegic agents are also useful for patients with significant ciliary spasm or photophobia. In the setting of IOP elevation, topical aqueous suppressants are first-line agents for pressure management (beta-blockers and alpha-agonists). Systemic carbonic anhydrase inhibitors and hyperosmotic agents (acetazolamide or mannitol) may be required if topical management fails to control the pressure. If this is the case, the patient will likely require surgical intervention.
It is important to note that carbonic anhydrase inhibitors are generally avoided in the treatment of pressure elevations because these agents may increase the sickling tendency in the anterior chamber of patients with sickle cell hemoglobinopathies by increasing aqueous acidity.[2] Also, systemic hyperosmotic agents may induce a sickle crisis in dehydrated patients with sickle cell disease and therefore should be avoided in these patients.
In addition, in those with sickle cell disease, adrenergic agonists with significant alpha1 activity (eg, apraclonidine [Iopidine]) are a concern because they may promote intravascular sickling by their vasoconstrictive and subsequent deoxygenating properties.[6]
Systemic and topical aminocaproic acid (ACA) has been recommended in various studies as a treatment option to avoid secondary hemorrhage in hyphema patients.[1][2][4][15] ACA is a derivative and analogue of the amino acid lysine and competitively inhibits plasmin, an important protein enzyme involved in fibrinolysis. If secondary hemorrhages are the result of lysis and retraction of a clot that has produced an occlusion of the traumatized vessel, then prevention of normally occurring clot lysis for 5-6 days should be advantageous to allow the injured blood vessel to more completely repair its integrity.[4] Tranexamic acid (TA) is another alternative. It is more potent than aminocaproic acid with fewer side effects.[16] Antifibrinolytics such as ACA and TA are not routinely used in the treatment of hyphemas, but they may be beneficial in patients at higher risk for rebleeding or other hyphema-associated complications.
If the IOP is not adequately controlled with medical management or the patient develops further ocular complications (corneal blood staining, rebleed), surgical treatment may be necessary.
Surgery
A majority of hyphemas will resolve with medical management alone. Approximately 5% of patients with a traumatic hyphema require surgery.[17] Patients can typically be monitored for the first 4 days with medical treatment alone to allow for spontaneous resolution. After that point, surgical intervention may be indicated in the setting of uncontrolled glaucoma, corneal blood staining, the persistence of a large or total hyphema, and active bleeding in the anterior chamber.[18] Uncontrolled intraocular hypertension is defined as greater than or equal to 50 mm Hg for more than 5 days, or more than 25 mm Hg for more than 24 hours in patients with sickle hemoglobinopathy despite maximal medical therapy.[1] Patients with sickle cell hemoglobinopathies and even those with sickle cell trait require surgical intervention if IOP is not controlled within 24 hours.[4] If a large hyphema results in complete visual obstruction in a pediatric patient, amblyopia could result. In these younger patients, early surgical intervention may be justified to avoid this visual complication.
Options for surgical intervention consist of anterior chamber irrigation and aspiration through a small incision (anterior chamber washout), hyphema evacuation with closed vitrectomy instrumentation, or clot irrigation with a filtering procedure (trabeculectomy). Additionally, an anterior chamber paracentesis can be performed for temporizing control of elevated IOP. If a total hyphema is present, it's possible that pupillary block could occur, and a peripheral iridectomy may be indicated during the time of surgery. An anterior chamber washout with irrigation and aspiration is commonly performed first.[2] If IOP remains uncontrolled, a trabeculectomy along with repeat anterior chamber washout is indicated. A trabeculectomy is generally not performed for smaller hyphemas, but it is used by some clinicians as the initial surgical approach (with concomitant peripheral iridectomy and anterior chamber washout).[4]
Postoperative hyphemas may be seen at the time of surgery or within the first 2-3 days after surgery. If bleeding occurs intraoperatively, it must be identified and coagulated if it does not cease on its own.[4]
Complications
- Obstruction of trabecular meshwork with associated IOP elevation
- Peripheral anterior synechiae
- Posterior synechiae
- Corneal blood staining: The American Academy of Ophthalmology's Pathology Atlas contains a virtual microscopy image of corneal blood staining.[19]
- Rebleeding: This can occur when the initial clot retracts and lyses, allowing for a second episode of bleeding. Rebleeds are generally more severe than the initial bleed and are more likely to lead to glaucoma, corneal blood staining, and synechiae formation.[6] Rebleeding has been reported to occur 3.5% to 38% of the time,[20] and the rate is likely 5%-10% overall. It usually occurs within the first 5 days after injury. Risk factors for rebleeding include hypotony or elevated IOP,[21] 50% or greater hyphema,[22] systemic hypertension,[23] and use of aspirin,[24] and Black patients are also at higher risk.[25]
- Pupillary block
- Amblyopia (pediatric patients)
Prognosis
Prognosis depends on the etiology and whether the patient developed an associated complication from the hyphema. These patients should be monitored closely to ensure adequate resolution of the hyphema without the development of visually debilitating complications. Additionally, patients with a history of ocular trauma require routine follow-up with gonioscopic examination because of the potential for development of angle-recession glaucoma.
Acknowledgements
All images are provided courtesy of the Krieger Eye Institute (KEI) at Sinai Hospital of Baltimore, along with Dr. Gregory Oldham.
Additional Resources
- Huffman JM. What is hyphema? American Academy of Ophthalmology. EyeSmart/Eye health. October 30, 2024. Accessed May 6, 2025. https://www.aao.org/eye-health/diseases/what-is-hyphema
- Porter D. What is a black eye? American Academy of Ophthalmology. EyeSmart/Eye health. September 10, 2024. Accessed May 6, 2025. https://www.aao.org/eye-health/diseases/black-eye
- Other EyeWiki articles:
References
- ↑ 1.0 1.1 1.2 1.3 Walton W, Von Hagen S, Grigorian R, Zarbin M. Management of traumatic hyphema. Surv Ophthalmol. 2002;47(4):297-334.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Basic and Clinical Science Course, Section 10. Glaucoma. American Academy of Ophthalmology; 2009-2010:114-116.
- ↑ Condon GP. Single-piece syndrome. Glaucoma Today. June 2011. https://glaucomatoday.com/articles/2011-june/single-piece-syndrome
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 Sheppard JD Jr et al. Hyphema. 2009. Medscape Reference.
- ↑ Nasrullah A, Kerr NC. Sickle cell trait as a risk factor for secondary hemorrhage in children with traumatic hyphema. Am J Ophthalmol. 1997;123(6):783-790.
- ↑ 6.0 6.1 6.2 6.3 Bazzaz S, Katz JL, Myers JS. Post-traumatic glaucoma. In: Shaarawy T, Sherwood MB, Hitchings RA, Crowston JG, eds. Glaucoma: Medical Diagnosis and Therapy. Elsevier; 2009:431-439.
- ↑ Coles WH. Traumatic hyphema: an analysis of 235 cases. South Med J. 1968;61(8):813-816.
- ↑ Irak-Dersu I et al. Glaucoma, Hyphema. 2009. Medscape Reference.
- ↑ 9.0 9.1 Watzke RC. Intraocular hemorrhage from wound vascularization following cataract surgery. Trans Am Ophthalmol Soc. 1974;72:242-252.
- ↑ Swan KC. Late hyphema due to wound vascularization. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1976;81(1):OP138-OP144.
- ↑ Swan KC. Hyphema due to wound vascularization after cataract extraction. Arch Ophthalmol. 1973;89(2):87-90. doi:10.1001/archopht.1973.01000040089001
- ↑ Leroux P, Rezkallah A, Kodjikian L, Loria O, Mathis T, Denis P. Recurrent hyphema after trabeculectomy: an atypical case of Swan syndrome. Int J Ophthalmol. 2021;14(10):1633-1635. doi:10.18240/ijo.2021.10.23
- ↑ Vijayalakshmi P, Shetty S, Jethani J, Uma Devi TB. Bilateral spontaneous hyphema in juvenile xanthogranuloma. Indian J Ophthalmol. 2006;56(1):45-46.
- ↑ Ehlers JP, Shah CP, eds. The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease. 5th ed. Lippincott Williams & Wilkins; 2008:20-22.
- ↑ Beiran I, Talmon T, Miller B. Characteristics and functional outcome of traumatic hyphema without routine administration of epsilon-aminocaproic acid. Isr Med Assoc J. 2002;4(11):1009-1010.
- ↑ Deans R, Noeel LP, Clarke WN. Oral administration of tranexamic acid in the management of traumatic hyphema in children. Can J Ophthalmol. 1992;27(4):181-183.
- ↑ Andreoli CM et al. Traumatic hyphema: epidemiology, anatomy, and pathophysiology. 2011. UpToDate.
- ↑ Wilson FM. Traumatic hyphema. Pathogenesis and management. Ophthalmology. 1980;87(9):910-919.
- ↑ Pathology Atlas. American Academy of Ophthalmology. September 16, 2016. Accessed January 4, 2017. https://www.aao.org/resident-course/pathology-atlas
- ↑ Volpe NJ, Larrison WI, Hersh PS, Kim T, Shingleton BJ. Secondary hemorrhage in traumatic hyphema. Am J Ophthalmol. 1991;112(5):507-513.
- ↑ Howard GM, Hutchinson BT, Frederick AR. Hyphema resulting from blunt trauma: gonioscopic, tonographic, and ophthalmoscopic observations following resolution of the hemorrhage. Trans Am Acad Ophthalmol Otolaryngol. 1965;69:294-306.
- ↑ Edwards WC, Layden WE. Traumatic hyphema. A report of 184 consecutive cases. Am J Ophthalmol. 1973;75(1):110-116.
- ↑ Fong LP. Secondary hemorrhage in traumatic hyphema. Predictive factors for selective prophylaxis. Ophthalmology. 1994;101(9):1583-1588.
- ↑ Ganley JP, Geiger JM, Clement JR, Rigby PG, Levy GJ. Aspirin and recurrent hyphema after blunt ocular trauma. Am J Ophthalmol. 1983;96(6):797-801.
- ↑ Spoor TC, Kwito GM, O'Grady JM, Ramocki JM. Traumatic hyphema in an urban population. Am J Ophthalmol. 1990;109(1):23-27.